Bio-Pharm: Multi-Tank
Multi-Tank CIP Systems for Decreased Cycle Times
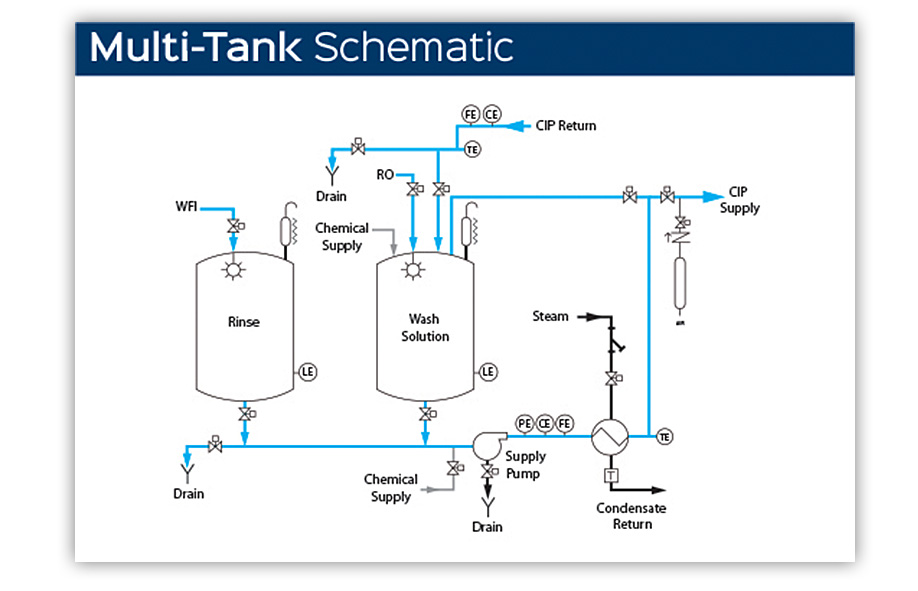
When time for cleaning is limited but space constraints and system portability are not a concern, bio-pharm manufacturing facilities may choose a multi-tank CIP system to decrease cycle times. Adding a second tank allows both the rinse and solution wash to be at the ready—no waiting for a single tank to refill, heat and charge with chemical following the initial rinse.
Sani-Matic Clean-In-Place (CIP) multi-tank systems are engineered to your specific plant application, layout and utility requirements and our in-house programming experts design each CIP program to optimize cycle times that get you back into production faster.
All Sani-Matic CIP Systems for bio-pharm applications are designed for critical cleaning and meet cGMP and ASME-BPE standards.
BENEFITS
Reduces risk of cross-contamination
Single-use source of cleaning solution and rinse water
Increases productivity
Dedicated rinse tank accumulates water between cleaning steps
Decreases wash cycle time
Standard Features
Allen-Bradley CompactLogix
Allen-Bradley PanelView Plus HMI
40 customizable cleaning cycle programs
Ethernet communication
Wetted parts: 316L stainless steel
UL listed, 304 stainless steel, NEMA 4X enclosure
Documentation Standard Features
Operation and maintenance manuals
Recommended spare parts list
Instrument lists
Instrumentation calibration procedures
Performance data
Material certificates
Weld qualification and inspection records
Inspection test results, reports and certificates
ASME data
Component catalog cut sheets
As-built assembly drawings
As-built process and instrumentation diagrams
As-built electrical drawings
PLC and HMI application files
Documentation Optional Features
Functional Specifications (FS)
Configuration Specification (CS)
Factory Acceptance Test (FAT)
Site Acceptance Test (SAT)
Installation and Operation Qualification (IQ/OQ)
Traceability matrix
ISA data sheets
Cleaning and passivation report
Riboflavin coverage test
Digital weld video record (Borescope)


